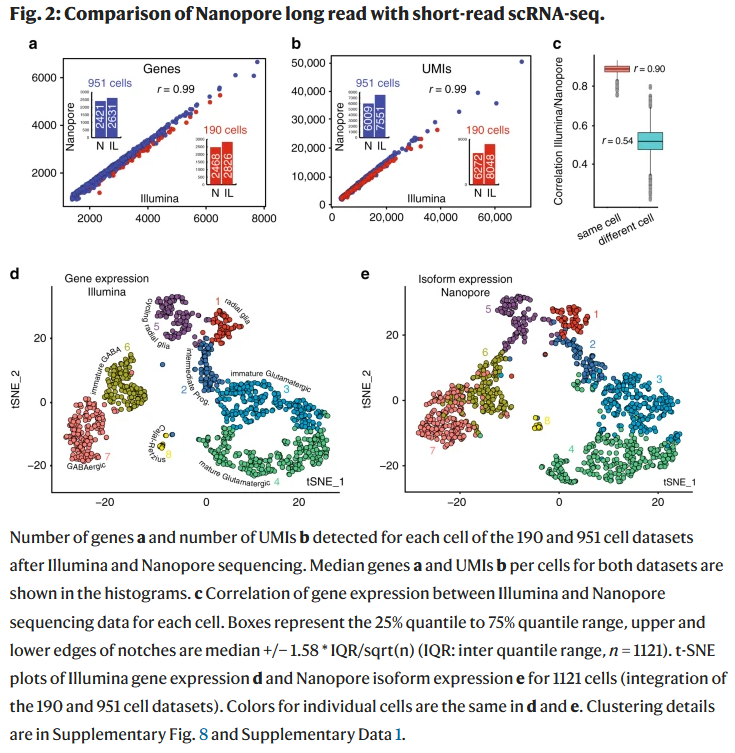
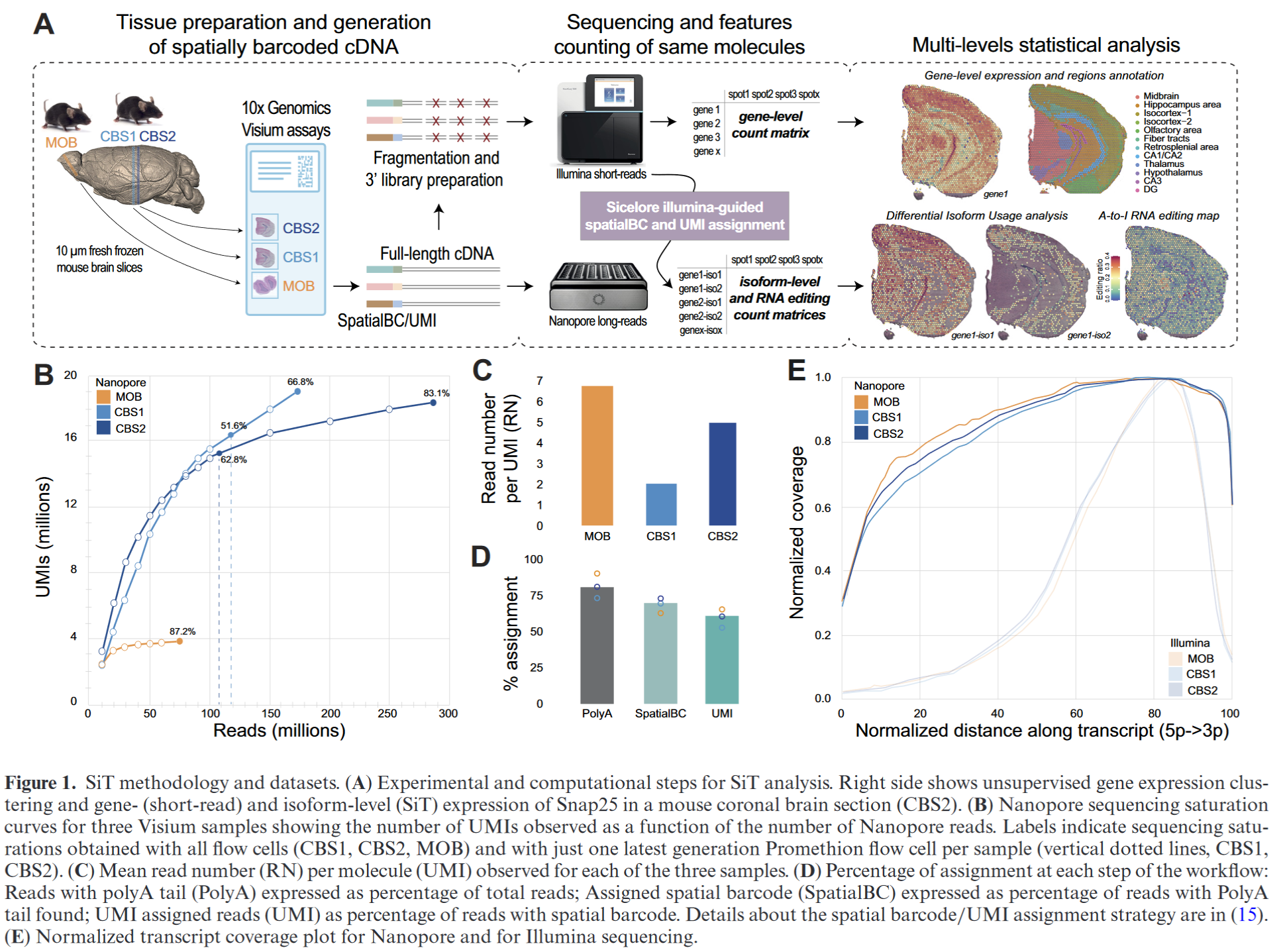
High throughput error corrected Nanopore single cell transcriptome sequencing
Droplet-based high throughput single cell sequencing techniques tremendously advanced our insight into cell-to-cell heterogeneity. However, those approaches only allow analysis of one extremity of the transcript after short read sequencing. In consequence, information on splicing and sequence heterogeneity is lost. To overcome this limitation, several approaches that use long-read sequencing were introduced recently. Yet, those techniques are limited by low sequencing depth and/or lacking or inaccurate assignment of unique molecular identifiers (UMIs), which are critical for elimination of PCR bias and artifacts. We introduce ScNaUmi-seq, an approach that combines the high throughput of Oxford Nanopore sequencing with an accurate cell barcode and UMI assignment strategy. UMI guided error correction allows to generate high accuracy full length sequence information with the 10x Genomics single cell isolation system at high sequencing depths. We analyzed transcript isoform diversity in embryonic mouse brain and show that ScNaUmi-seq allows defining splicing and SNVs (RNA editing) at a single cell level.